The last decade saw the rise of the biopharmaceutical industry and the fast adoption of biological products. The industry carries a wide range of products, from vaccines to personalized therapies or targeted products and specialty medicines for many orphan diseases. The industry had to pivot from a traditional model of processing block-buster compounds to limited biopharmaceutical production runs and specific manufacturing requirements to cater to these products.
Key facts
12 years
The average time it takes to develop a drug

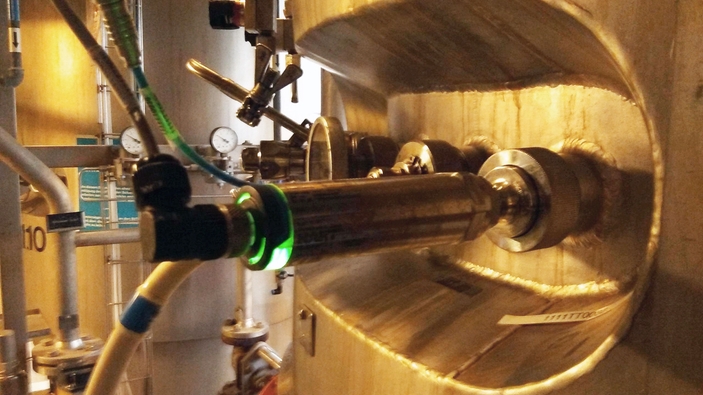
Equipment sterilization in medical autoclaves
Medical autoclaves rely on precise and reliable temperature measurements to comply with process requirements. New sensor technology automates recalibrations, reducing risk between intervals and providing audit-proof batch certification.
Our expertise in the field
Self-calibrating temperature measurement technology can minimize the risk of incorrect measurements and improve process safety and regulatory compliance (FDA) in pharmaceutical applications with continuous process verification (GMP). Automated documentation helps effortlessly build a repeatable, traceable record database for audits and regulatory authorities.
- The self-calibrating hygienic iTHERM TrustSens TM371 lowers risk and drives automation in regulated processes with fully automated process documentation and built-in memory for 350 calibration events.
- The Heartbeat Technology offers full instrument diagnostics, monitoring and verification, effectively eliminating the risk of undetected non-conformities.

Instrumentation designed according to ASME-BPE
The ASME-BPE has brought some degree of standardization to the equipment used in biopharmaceutical processing and includes best-practices for enhancing product purity and safety while maintaining high levels of hygiene. Reflecting the innovative nature of the industry, it is reviewed regularly. Companies that rigorously apply ASME-BPE can often achieve higher production efficiencies, lower development and manufacturing costs, and increase quality and safety, while complying with regulations.
Our expertise in the field
For a system or component to be ASME BPE-compliant, adherence to all applicable parts of the standard is required. The stringent requirements of BPE often mean that only devices specifically designed according to standard can achieve full compliance. Endress+Hauser offers a complete portfolio of instruments that are designed and manufactured according to ASME BPE, such as:
- Promass P 100 Coriolis flowmeter is an ASME BPE-compliant specialist with an ultra-compact transmitter. It delivers total performance on the smallest footprint. They are designed for applications in sterile environments where space is a premium.
- Digital oxygen sensor Memosens COS22E Memosens 2.0 hygienic, amperometric oxygen sensor for the food and life sciences industries
- Absolute and gauge pressure Cerabar PMP51 Digital pressure transmitter with welded metal sensor for measurement in gases, steam or liquids
- Vibronic Point level detection Liquiphant FTL50H
Compact vibration point level switch for liquids predominantly in the food and life sciences industry - Sanitary digital thermometer iTHERM TM411, a hygienic temperature measurement instrument for optimum product quality, process safety, and efficiency

The next generation of single-use instruments
Disposable equipment is now estimated to be present in 85% of pre-market manufacturing, and its usage is also increasing for the commercial production of biological medicines. Since the equipment is sterile and ready to use, there is no additional need for cleaning, sterilizing, or validating any of its components. Single-use technology results in a simplified, more flexible production and faster time to market biopharmaceuticals.
Our expertise in the field
Single-use instrumentation must offer equivalent reliability and measurement accuracy as their reusable counterparts while still providing key attributes such as minimized hold-up volume, an ergonomic design, no required calibration, and the ability to optimize costs. Endress+Hauser has risen to the challenge, collaborating with leading technology providers to extend our portfolio with an equivalent single-use offering for upstream and downstream process applications.
- Raman Rxn-46 probe connection to BioPAT® Spectro for Ambr® 15
and Ambr® 250 by Sartorius

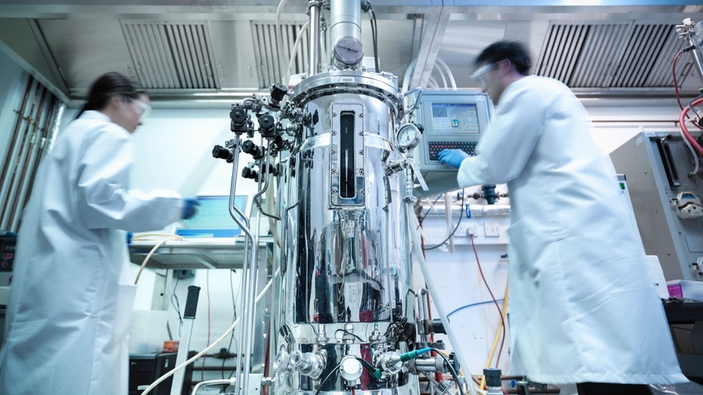
Advanced analytics for process control in bioreactors
In a bioreactor, it is essential to keep the dissolved oxygen and pH at an optimal level at all times. Cell density and metabolites should also be monitored for a better insight into the process. The concentration of metabolites is typically measured off-line with time-consuming techniques and therefore requires sampling with the associated risks.
Our expertise in the field
Raman spectroscopy is an ideal solution for fast and non-destructive measurement. Our Raman solutions can monitor several parameters simultaneously with one single probe installed directly in-line. The complete upstream package enables real-time and optimized control of the process leading to reduced time between batches, limited risk of contamination and increased yield.
- Kaiser Raman Rxn analyzer is designed for use in analytical laboratories with model transfer capabilities.
- Raman Rxn-10 probe with Raman optic system for single use, a bioprocess probe with sterile, disposable fittings for single-use bioreactors.
Benefits
From our ASME-BPE compliant portfolio to our advanced analytical technology and calibration services, we are committed to helping you improve your biopharmaceutical manufacturing process, getting your product to market faster while safeguarding product quality and mitigating risk.
Key facts
35
fermentation batches until ROI of a single channel Kaiser Raman Rxn system
Key facts
2 months
faster time to market when streamlining your projects for the biopharmaceutical manufacturing processes together with our industry experts
Key facts
10%
cost reduction thanks to preventive maintenance concepts
How we can help
Endress+Hauser is committed to addressing the challenges of the biopharmaceutical industry. By integrating consistent and comparable data with our measurement technology, we enable the rapid detection of process changes and therefore warrant consistent quality attributes of your product. With our complete portfolio that meets the biopharmaceutical industry standards, we support you in releasing your product to the market faster while safeguarding high-quality attributes.
- Complete ASME BPE-compliant instrument portfolio that accurately monitors critical process conditions from lab to process.
- Full solutions specially designed for biopharmaceutical manufacturing, including Process Analytical Technology (PAT) for real-time control and optimization.
- Expert consulting in process scale-up from pilot to automated production units.
- Calibration services and predictive maintenance that ensure full compliance with minimal interruptions.
- Expertise in project planning and engineering, facilitating plant setup with early standardization.